
Radiation
Alpha decay is a radioactive process in which a particle with two neutrons and two protons is ejected from the nucleus of a radioactive atom. The particle is identical to the nucleus of a helium atom.
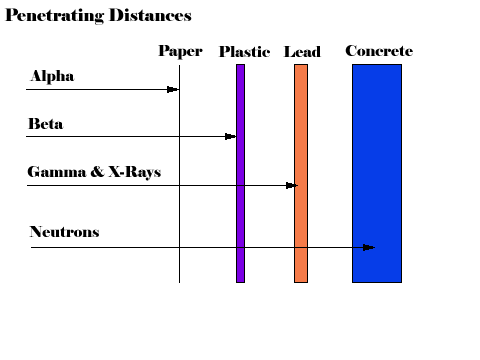
Because alpha particles contain two protons, they have a positive charge of two. Further, alpha particles are very heavy and very energetic compared to other common types of radiation. Typical alpha particles will travel no more than a few centimeters in air and are stopped by a sheet of paper.
Beta particles have a single negative charge and weigh only a small fraction of a neutron or proton. As a result, beta particles interact less readily with material than alpha particles. Depending on the beta particles energy (which depends on the radioactive atom), beta particles will travel up to several meters in air, and are stopped by thin layers of metal or plastic.
After a decay reaction, the nucleus is often in an excited state. This means that the decay has resulted in producing a nucleus which still has excess energy to get rid of. Rather than emitting another beta or alpha particle, this energy is lost by emitting a pulse of electromagnetic radiation called a gamma ray. The gamma ray is identical in nature to light or microwaves, but of very high energy.
Like all forms of electromagnetic radiation, the gamma ray has no mass and no charge. Gamma rays interact with material by colliding with the electrons in the shells of atoms. They lose their energy slowly in material, being able to travel significant distances before stopping. Depending on their initial energy, gamma rays can travel from 1 to hundreds of meters in air and can easily go right through people.