|
To Print: Click your browser's PRINT button.
NOTE: To view the article with Web enhancements, go to: http://www.medscape.com/viewarticle/487585 High
Incidence of Appropriate and Inappropriate ICD Therapies in Children
and Adolescents with Implantable Cardioverter Defibrillator Thomas Korte; Harald Köditz; Michael Niehaus; Thomas Paul; Jürgen Tebbenjohanns Pacing Clin Electrophysiol 27(7):924-932, 2004. © 2004 Blackwell Publishing Posted 08/20/2004 Abstract and IntroductionAbstractAppropriate and inappropriate therapies of implantable cardioverter defibrillators have a major impact on morbidity and quality of life in ICD recipients, but have not been systematically studied in children and young adults during long-term follow-up. ICD implantation was performed in 20 patients at the mean age of 16 ± 6 years, 11 of which had prior surgical repair of a congenital heart defect, 9 patients had other cardiac diseases. Implant indications were aborted sudden cardiac death in six patients, recurrent ventricular tachycardia in 9 patient, and syncope in 5 patients. Epicardial implantation was performed in 6 and transvenous implantation in 14 patients. Incidence, reasons and predictors (age, gender, repaired congenital heart disease, history of supraventricular tachycardia, and epicardial electrode system) of appropriate and inappropriate ICD therapies were analyzed during a mean follow-up period of 51 ± 31 months range 18-132 months. There were a total 239 ICD therapies in 17 patients (85%) with a therapy rate of 2.8 per patient-years of follow-up. 127 (53%) ICD therapies in 15 (75%) patients were catagorized as appropriate and 112 (47%) therapies in 10 (50%) patients as inappropriate, with a rate of 1.5 appropriate and 1.3 inappropriate ICD therapies per patient-years of follow-up. Time to first appropriate therapy was 16 ± 18 months. Appropriate therapies were caused by ventricular fibrillation in 29 and ventricular tachycardia in 98 episodes. Termination was successful by antitachycardia pacing in 4 (3%) and by shock therapy in 123 episodes (97%). Time to first inappropriate therapy was 16 ± 17 months. Inappropriate therapies were caused by supraventricular tachycardia in 77 (69%), T wave oversensing in 19 (17%), and electrode defect in 16 episodes (14%). It caused shocks in 87 (78%) and only antitachycardia pacing in 25 episodes (22%). No clinical variable could be identified as predictor of either appropriate or inappropriate ICD therapies. There is a high rate of ICD therapies in young ICD recipients, the majority of which occur during early follow-up. The rate of inappropriate therapies is as high as 47% and is caused by supraventricular tachycardia and electrode complications in the majority of cases. Prospective trials are required to establish preventative startegies of ICD therapies in this young patient population. IntroductionThe implantable cardioverter defibrillator has been shown to be remarkably effective in preventing sudden cardiac death and total mortality in both adults and children with life threatening ventricular tachyarrhythmia.[1-5] Recent studies have shown favorable results for defibrillators implanted in high risk patients with prophylactic indication.[6-7] Since changes in the socioeconomic climate force justification of the use of expensive medical intervention,[8] potential benefits of defibrillator therapy have to be characterized focusing on softer endpoints such as cardiac morbidity and quality-of-life. A major issue of ICD therapy is the high incidence of both appropriate and inappropriate ICD therapies[9-12], which have a major impact on morbidity and quality-of-life in both adult and young ICD recipients.[13,14] A number of studies have focused on antiarrhythmic drug treatment, advanced detection criteria, and ablation procedures as prevention strategies of ICD therapies.[15-23] Little is known about the incidence of ICD therapies in children and young adults. In this population, size, growth, prior cardiac surgery with complex anatomy, high incidence of supraventricular tachycardia,[24] and a particularly high impact of ICD discharges on quality-of-life[25] may warrant specific considerations in the use of the ICD. This is a single center study to analyze the incidence, reason, and time in follow-up of appropriate and inappropriate ICD therapies in children and young adults after epicardial or transvenous ICD implantation. MethodsPatient Population and ICD ImplantationThe study population included 20 consecutive children (<18 years of age) and young adults with repaired congenital heart disease who received an epicardial (Fig. 1) or transvenous implantable cardioverter defibrillator for malignant ventricular arrhythmia at the Hannover Medical School, between October 1990 and May 1999 (Table I). Cardiac diagnoses included repaired congenital heart disease in 11 (55%) patients, a long QT syndrome in 4 (20%) patients, primary ventricular fibrillation in 2 (10%) patients, and arrhythmogenic right ventricular dysplasia, idiopathic dilated cardiomyopathy, and hypertrophic cardiomyopathy in 1 (5%) patient, respectively. The presenting arrhythmia was cardiac arrest in 8 (40%) patients, ventricular tachycardia in 7 (35%) patients and syncope in 5 (25%), patients. Indication for ICD implantation followed the ACC/AHA guidelines.[26] Invasive electrophysiological study was performed prior to ICD implantation with exception of the patients presenting with long QT syndrome. Sustained ventricular tachycardia (VT) or ventricular fibrillation (VF) was inducible in 12 of 16 (75%) patients by triple programmed stimulation of the right ventricle at two sites. Defibrillation threshold testing and measurement of R wave amplitude and pacing threshold of the rate-sensing/pacing leads were performed intraoperatively in the standard fashion.[27,28] 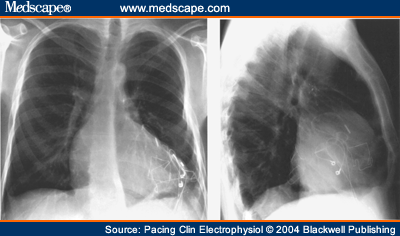 Figure 1. Chest X ray of a 23-year-old patient with stauts post surgical repair of a double outlet left ventricle, interventional closure of a patent ductus arteriosus and implantation of an epicardial ICD system. Device ProgrammingDevice programming was dependent on the clinical arrhythmia, results of the electrophysiological study, and cardiac function, as has been described by others.[27,29] The zone for VT detection was programmed at least 10 beats/min below the documented or induced ventricular tachycardia, and 20 beats/min below an induced or spontaneous polymorphic ventricular tachycardia. In patients with only documented ventricular fibrillation, the zone for tachycardia detection was programmed to >/= 180 beats/min. In case of a spontaneous or induced hemodynamically tolerated ventricular tachycardia, two detection zones were programmed and antitachycardia pacing was programmed as first therapy in the lower frequency zone followed by shock therapy. In the high frequency zone, only shock treatment was programmed. Advanced detection algorithms were not programmed at first hospital discharge. Antiarrhythmic drug treatment, if present, was not changed before ICD implantation and before hospital discharge after implantation. Follow-up and Management StrategyFollow-up began the day of hospital discharge after first ICD implantation. The patients were routinely seen in the outpatient clinic of the implanting center 4 weeks after implantation and then every 3 months. Patients experiencing a shock from the device or symptoms compatible with a ventricular or supraventricular tachyarrhythmia without a shock, were instructed to return before the scheduled follow-up visit for interrogation of the device and analysis of the diagnostic information and any stored electrograms. Because of the limitations of storage capability of the devices, electrograms corresponding to some arrhythmia episodes had been replaced by subsequent events and were therefore unavailable for review. Data are presented only for those episodes in which ventricular electrograms were available for analysis. Pertinent information was further obtained by telephone contact with patients, their families, or private physicians. No patient was lost to follow-up. Management for Device Responses for VT. For patients in whom electrogram analysis indicated VT as the rhythm leading to device response, interventions were individualized based on analysis of the stored event. If the initial therapy (antitachycardia pacing or shock) was successful, no alterations in the treatment algorithm were implemented. For patients in whom a shock was required after antitachycardia pacing had failed to terminate the arrhythmia, efforts were made to alter the antitachycardia pacing algorithm to improve the efficacy of antitachycardia pacing for spontaneous VT termination. Management of Device Response for Non-VT Rhythms. If a device response for a non-VT rhythm was suggested from analysis of stored electrograms, several changes in tachycardia detection criteria and/or antiarrhythmic drug regimen were usually implemented in an effort to prevent either a recurrence of the arrhythmia or recurrent device response for the arrhythmia. Criteria of Arrhythmia Diagnosis Using Stored Ventricular ElectrogramsVentricular Tachycardia. A diagnosis of VT was based primarily on a change in ventricular electrogram morphology during tachycardia relative to that recorded during baseline rhythm.[30] Differences in electrogram morphology were considered present if, upon visual inspection, there was any alteration in the number or polarity of individual electrogram components. Atrial Fibrillation. The presence of variability in RR intervals of more than 50 ms for three or more of 10 consecutive intervals with no change in the electrogram morphology relative to the baseline rhythm, was used to classify a rhythm as atrial fibrillation.[30,31] Supraventricular Tachycardia. A tachycardia without the RR interval variability described for atrial fibrillation and with no change in the electrogram morphology relative to the baseline rhythm was classified as supraventricular tachycardia. The absence of an atrial recording lead in most patients precluded a more specific arrhythmia diagnosis, although a history of atrial tachycardia, atrial flutter, or activities consistent with sinus tachycardia before device response were helpful in establishing a probable diagnosis. Lead and Device Complications. High frequency, high amplitude signals recorded from the sensing leads consistent with an intermittent make-break phenomenon were attributed to disruption of the rate-sensing lead system. The diagnosis was confirmed by an abnormal rat-sensing/pacing lead impedance recorded during real-time measurements and by reproducing the electrical artefact on a real-time electrogram recording channel with manipulation of the generator. T wave oversensing was diagnosed by analysis of endocardial electrograms and real-time endocardial recordings (Fig. 2). 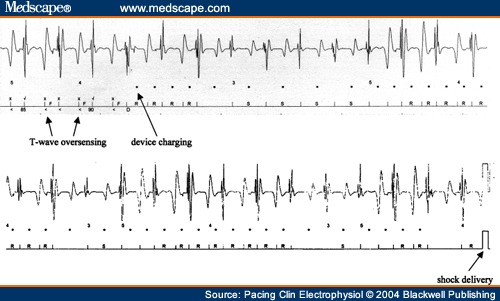 Figure 2. Inappropriate ICD shock due to T wave oversensing in a 12-year-old girl with long QT syndrome and survived sudden cardiac death. Since T wave oversensing continued despite device reprogramming, a repositioning of the right ventricular electrode had to be performed. Electrogram analysis was performed by two investigators independently (T.K., M.N.). StatisticsThe rates of appropriate and inappropriate ICD therapies were determined by dividing the number of therapies experienced by the total number of patient-years of follow-up for both individual patients and for groups. Continuous variables are expressed as mean ± SD. The probabilities of freedom from appropriate and inappropriate ICD therapies were estimated by the method of Kaplan and Meier. As predictors of the number of appropriate and inappropriate ICD therapies, age (dichrotomized at </= or > 14 years), gender, repaired congenital heart disease, history of supraventricular tachycardia, and epicardial electrode system were analyzed, making use of the unpaired Student's-test. A P value 0.05 was considered statistically significant. ResultsICD Implantation, Programming and Follow-upSuccessful ICD implantation was performed in 6 (30%) patients with epicardial lead system and in 14 (70%) patients with transvenous lead system. In five of the six patients with thoracotomy, the epicardial system was implanted due to the complex anatomy of repaired congenital heart disease (Table I). In one patient an epicardial system was implanted due to high defibrillation threshold of the endocardial system. There was no perioperative mortality. All patients initially received a single chamber ICD system (12 Cardiac Pacemakers Inc, St. Paul, MN USA; 7 St. Jude Ventritex Inc, Sunnyvale, CA, USA; 1 Medtronic Inc, Minneapolis, MN, USA). In 16 (80%) patients one detection zone with a lower detection cut off of 206 ± 10ms (range 190-220) and only shock therapy was programmed. In four (20%) patients, two detection zones with a lower zone for both antitachycardia pacing and shock therapy, a lower detection cut off at 176 ± 8 ms (range 165-180) and a second zone for only shock therapy with a lower detection cut off at 208 ± 15 ms (range 200-230) was programmed. Mean follow-up period of the patients was 51 ± 31 months (range 18-132). Eighteen (90%) patients were followed for at least 24 months and 11 (55%) patients were followed for >/= 36 months. In 4 (20%) patients 1 device exchange, in 1 (5%) patients 2 device exchanges, in 2 (10%) patients 3 device exchanges, and in 1 patient 4 device exchanges for battery depletion were performed. System revision for electrode complications was required in 8 (40%) patients due to an insulation defect in 4 (20%) patients, T wave- or myopotential-oversensing in 2 (10%) patients, and lead dislodgement in 2 (10%) patients. Four (20%) patients died 39 ± 2 (range 25-70) months after implantation. Reasons were congestive heart failure in two patients and recurrent grand mal attacks in one patients. Heart transplantation was performed in one patient, who died 7 months later due to transplant failure. The clinical characteristics of the patients are summarized in Table I. Appropriate and Inappropriate ICD TherapiesSeventeen of the 20 (85%) patients received 239 ICD therapies (Table I) during follow-up with a therapy rate of 2.8 per patient-years of follow-up. Of these therapies, 127 (53%) in 15 patients (75%) were classified as appropriate with a rate of 1.5 appropriate therapies per patient-year of follow-up. The time to first appropriate ICD therapy was 16 ± 18 (range 1-72) months (Fig. 3). One hundred twelve (47%) treated episodes in 10 (50%) patients were classified as inappropriate with a rate of 1.3 inappropriate ICD therapies per patient-year of follow-up. The time to first inappropriate ICD therapy was 16 ± 17 (range 3-49) months (Fig. 3). 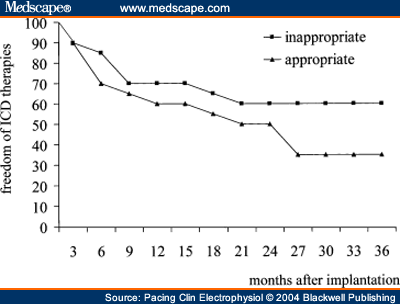 Figure 3. Kaplan-Meier curves depicting freedom from appropriate ( Device Responses for VT and VF. Twelve (60%) patients received 96 appropriate ICD therapies for ventricular tachycardia, which were terminated by antitachycardia pacing in four (4%) episodes and by shock therapy in 92 (96%) episodes (Fig. 4). Since the first appropriate therapy was a shock in all patients, the time to first appropriate shock also was 16 ± 18 (range 1-72) months. Five (25%) patients had 29 episodes of ventricular fibrillation, which were all terminated by first shock therapy. Antiarrhythmic therapy was changed in six (30%) patients. The algorithm of antitachycardia pacing was changed in three (15%) patients. In one patient with repaired congenital heart disease and recurrent shocks for VT, radiofrequency ablation with the non contact mapping technique (Ensite-Mapping, Medtronic, Inc) was performed. In one patient with long QT syndrome and recurrent shocks for sustained VF, left-sided stellectomy was performed and the ICD system was up graded to a dual chamber device to allow constant atrial pacing. The patient has not experienced further ICD discharges during a follow-up period of 41 months. One patient with recurrent adequate shocks for fast VT has not experienced shock recurrence after change of antiarrhythmic medication, but has acquired severe psychiatric problems due to ICD shocks and needs long-term psychiatric treatment. 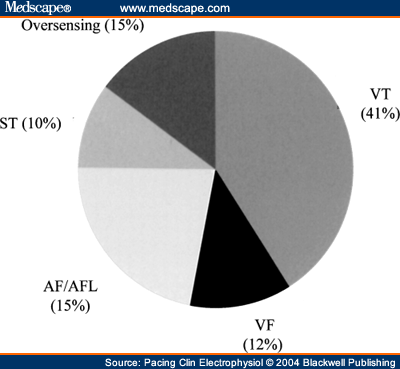 Figure 4. Pie chart with distribution of causes of appropriate and inappropriate ICD therapies. VT = ventricular tachycardia, VF = ventricular fibrillation; AF = atrial fibrillation; AFL = atrial flutter; ST = sinus tachycardia; Oversensing = oversensing of all causes. Device Responses for Supraventicular Tachyarrhythmia. Eight (40%) patients received 77 (69%) inappropriate ICD therapy for supraventricular tachyarrhythmia with atrial fibrillation in three (15%) patients, atrial tachycardia or type-II atrial flutter in 4 (20%) patients and sinus tachycardia in one (5%) patient, respectively (Fig. 4). Preventative strategy for further inappropriate therapies was change of antiarrhythmic medication in six (30%) patients. In three (15%) patients the lowest detection zone was altered. Device Responses for Lead or Device Complications. Lead and device related complications caused 35 (30%) inappropriate therapies in five (25%) patients (Fig. 4). T wave oversensing was the reason for 19 (17%) inappropriate episodes in two (10%) patients. The time to first inappropriate therapy for lead failure (i.e., time to first shock for lead failure, since all first therapies were shocks) was 20 ± 21 (range 2-46) months. Five of the six patients with lead failure were children, lead failure occurred in four endocardial and two epicardial lead systems. Lead fracture, dislodgements, or insulation defects of leads or adapters caused 16 (14%) inappropriate episodes in four (20%) patients. In all patients with hardware problems as cause of inappropriate therapies, operative lead revision had to be performed. Predictors for Appropriate and Inappropriate ICD Therapies. None of the analyzed variables, i.e., age, gender, repaired congenital heart disease, history of supraventricular tachycardia, or epicardial electrode system was able to predict the occurrence of either appropriate or inappropriate ICD therapies per patient-year of follow-up (P > 0.05, respectively). The analysis was performed despite small patient numbers in some of the subgroups. DiscussionMain FindingsThis single-center study is the first to demonstrate the high incidence of ICD therapies in 85% of children and adolescents during long-term follow-up after cardioverter defibrillator implantation. Fifty-three percent of ICD therapies in 15 (75%) patients were catagorized as appropriate, 47% of therapies in 10 (50%) patients were inappropriate, 69% of which were caused by supraventricular tachyarrhythmia and 31% by hard ware problems. No clinical variable was able to predict either appropriate or inappropriate ICD therapies. Appropriate ICD ResponsesIncidence. The high efficacy of the implantable cardioverter defibrillator in the prevention of sudden cardiac death has been well demonstrated in large adult ICD populations.[1,10,32] Efficacy and safety of the implantable defibrillator in young patients, in whom issues of size, growth, and prior cardiac surgery pose a particular challenge to ICD therapy, has only been addressed in a few, small series. Paul et al.[25] were one of the first to report on successful ICD implantation in four children, three of which experienced appropriate ICD discharges during a mean of 13 months of follow-up. Kron et al.[4] reported on appropriate ICD therapies in 10 of 17 young patients with only nonthoracotomy ICD systems during a mean follow-up period of only 7.9 months. Hamilton et al.[3] reported on 60% of 11 young ICD recipients receiving at least one appropriate ICD discharge by 1.5 years after implantation. None of these authors report on failure of an ICD system in termination of VT/VF. Our data confirm the high incidence of appropriate ICD discharges in this young patient population and the high efficacy in the prevention of sudden cardiac death. All of the 127 spontaneous VT and VF episodes were terminated by ICD therapy. In particular, ICD therapy is demonstrated to be effective in appropriately selected high risk patients presenting with syncope. Indeed, four of five patients presenting with syncope and selected for device implantation based on electrohysiological study and family history, have had appropriate clinical discharges. Predictors and Prevent Strategies. None of the analyzed clinical variables, i.e., age, gender, repaired congential heart disease, history of supraventricular tachycardia, and epicardial electrode system was able to predict the occurrence of appropriate ICD discharges. Frequent appropriate ICD discharges have a high impact on morbidity and quality-of-life in ICD patients and are the most frequent cause of unscheduled rehospitalisation after ICD implantation.[13,33] In our study, two patients with frequent appropriate ICD discharges needed intensive psychiatric treatment. Inappropriate ICD ResponsesIncidence. The incidence of inappropriate ICD therapies has been well analyzed in large series of adult recipients and is still as high as 20%-30%[9,34], Nunain et al.[34] reported on 21% of patients receiving inappropriate therapies due to supraventricular tachycardia. Weber et al.[29] reported on atrial fibrillation as the most frequent cause (39%), followed by sinus tachycardia in 30%, and lead oversensing due to fractures and insulation defects in 24% of patients studied. Only very limited data is available about the incidence of inappropriate ICD therapies in the young. Love et al.[35] found inappropriate ICD discharges in only 15% of young patients, but it is unclear if inappropriate antitachycardia pacing was included in the analysis. Hamilton et al.[3] reported on only 39% of young ICD recipients without inappropriate ICD discharges after 3 years of follow-up, inappropriate therapies were mainly caused by sinus tachycardia. Our data confirm the particularly high incidence of inappropriate ICD therapies in children and adolescents. As in adult series, the main cause was supraventricular tachycardia, followed by lead complications. As in other studies[3,29], most inappropriate therapies occurred during early follow-up, i.e., within the first 18 months after ICD implantation. Predictors and Preventative Strategies. As for appropriate ICD therapies, we and others were unable to identify any clinical variable that was able to predict the occurrence of inappropriate ICD discharges. Inappropriate ICD discharges have a high impact on morbidity and quality-of-life in ICD patients and aside from appropriate discharges they are a frequent cause of unscheduled rehospitalization after ICD implantation.[13] A number a preventative strategies for inappropriate ICD discharges have been studied in adult series. Advanced detection criteria for improved specificity in the detection of ventricular tachycardia in the lower tachycardia detection zone have been studied for both single and dual chamber ICD systems.[16-21,36-38] Neuzner et al.[36] reported on improved sensitivity and specificity with the use of the rate stability criterion and sudden onset criterion. We recently reported [18] on improved sensitvity and specificity making use of a new morphology detection criterion in adult patients with single-lead system. The first data is available about the specificity of dual chamber detection algorithms [19-21,38] with a proposed further improvement of specificity of VT detection. No data is available about the use of advanced detection criteria in young ICD recipients. In our study, high cut-off rates for VT detection were used to avoid spurious shocks for sinus tachycardia. In 80% of the patients, only one detection zone was used. All patients received single lead systems. Thus the use of advanced single and double chamber detection algorithms may be limited in the young patient population, but they have to be addressed in future prospective studies. Treadmill testing was not performed in all patients in this study and might be helpful for programming the lower detection zone for avoidance of shocks for sinus tachycardia. No systematic data is available about the use of concomittent antiarrhythmic medication in both adult and young patient populations. In the present study 45% of the patients were discharged from the hospital on either β-blocker or a Class III or I antiarrhythmic drug. Future prospective studies have to address the efficacy of antiarrhythmic drugs to avoid appropriate and inappropriate ICD therapies in this patient population. We recently reported[23] on ablation procedures for atrial fibrillation and type-I atrial flutter for the avoidance of inappropriate therapies in adult ICD recipients and showed a significant reduction of spurious therapies. The often complex anatomy and complex atrial arrhythmia may pose a particular challenge on ablation procedures in young patients, but it may be considered in selected patients using moderne three-dimensional mapping systems.[24] In adult populations, the incidence of lead complications is as high as 1%-5% in both transvenous and endocardial ICD systems[11,12]. Particularly lead fractures, insulation defects, and connector problems are often diagnosed on occasion of inappropriate ICD therapies due to sensing of electrical artefacts.[12] No systematic data about lead complications in the young patient population is available. In the present study, six patients received spurious therapies for lead problems, which had to be surgically revised in all patients. Issues of size, growth, and complex anatomy after prior cardiac surgery pose a particular challenge on lead technology and implant techniques in the young. Five of the six patients with lead failure were children, lead failure occurred in four endocardial and two epicardial lead systems. But the numbers in this study are too small to show that either skeletal growth or lead system used have directly influenced the occurrence of inappropriate therapies due to lead failure. Efforts have to be made to improve technology, particularly in this patient population, in order to minimize inappropriate therapies and the high rate of lead revisions in both epicardial and transvenous systems. Prospective multicenter studies are mandatory to study preventative strategies for both appropriate and inappropriate ICD therapies in children and young adult ICD recipients, since ICD interventions have a particular impact on morbidity and quality-of-life in this patient population. LimitationsThis single center study was not able to prospectively and systematically analyze preventative strategies in this selected patient population. All patients received single lead ICD systems, which limited the clear differentiation of atrial fibrillation and supraventricular tachycardia as the cause of inappropriate shocks by analysis of stored endocardial electrograms. ConclusionThere was a high incidence of appropriate and inappropriate ICD therapies in children and adolescents with epicardial or transvenous ICD system during long-term follow-up. Most of the first appropriate and inappropriate ICD therapies occurred during early follow-up and could not be predicted by clinical variables. Future studies are mandatory to prospectively evaluate preventative strategies of both appropriate and inappropriate ICD interventions in this young group of ICD recipients.
TablesTable I. Characteristics of Patients and Incidence of Appropriate and Inappropriate ICD Therapies
References
Reprint Address Thomas Korte, M.D. Dept. of Cardiology and Angiology, Medical School Hannover, Carl-Neuberg-Str. 1, 30625 Hannover, Germany. Fax: ++49-511-532-8475; e-mail: korte.thomas@mh-hannover.de Thomas Korte, Harald Köditz*, Michael Niehaus, Thomas Paul†, and Jürgen Tebbenjohanns
From the *Department of Cardiology and Pediatric Cardiology, Medical School Hannover, Hannover, Germany, and the †Department of Pediatric Cardiology, Medical University of South Carolina, Charleston, SC |